Food Labelling - Archive Page
CAUTION: This is an archive page and is retained for historic interest only. Information contained here should not be used for developing labels. Links may not be correct. For information on the current requirements, see: Food Labelling
On this page:
- EU Law - Description of the legislation controlling food labelling in the EU with links to EU documents
- Development of New EU Controls - Information and links relating to the new labelling controls
- UK Law - Links to the UK legislation and guidance notes implementing the EU law
EU Law
Agreed EU-wide controls on food labelling were introduced with Directive 79/112 in 1979. Additional controls have been added and amendments introduced to produce a complex array of labelling requirements. In 2000, the original 1979 Directive and its amendments were consolidated into a single new Directive - Directive 2000/13/EC. Additional Directives relating to labelling have also been adopted. This situation is complex but can be summarised in the following diagram:
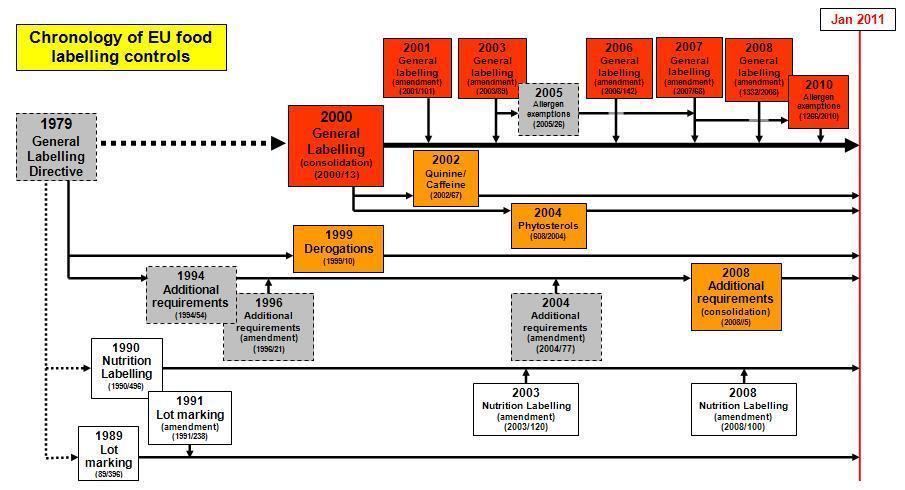
The following links are to the current versions of the EU directives on the EU web site (but see also links to the consolidated texts given below):
- Directive 2000/13/EC of the European Parliament and of the Council of 20 March 2000 on the approximation of the laws of the Member States relating to the labelling, presentation and advertising of foodstuffs
As corrected by:- Corrigendum to Directive 2000/13/EC of the European Parliament and of the Council of 20 March 2000 on the approximation of the laws of the Member States relating to the labelling, presentation and advertising of foodstuffs [Giving the English langauge version of Annex III]
As amended by:
- Commission Directive 2001/101/EC of 26 November 2001 amending Directive 2000/13/EC of the European Parliament and of the Council on the approximation of the laws of the Member States relating to the labelling, presentation and advertising of foodstuffs [Giving a definition of meat]
- Directive 2003/89/EC of the European Parliament and of the Council of 10 November 2003 amending Directive 2000/13/EC as regards indication of the ingredients present in foodstuffs [Including extra requirements for allergen labelling]
- Commission Directive 2006/142/EC of 22 December 2006 amending Annex IIIa of Directive 2000/13/EC of the European Parliament and of the Council listing the ingredients which must under all circumstances appear on the labelling of foodstuffs [Extending the list of specified allergens]
- Commission Directive 2007/68/EC of 27 November 2007 amending Annex IIIa to Directive 2000/13/EC of the European Parliament and of the Council as regards certain food ingredients (OJ L310, 28.11.2007, page 11) [Providing a new Annex IIIa which gives permanent exemptions to some of those ingredients previously given temporary exemption by Directive 2005/26 (see below)]
- Regulation (EC) No 1332/2008 of the European Parliament and of the Council of 16 December 2008 on food enzymes and amending Council Directive 83/417/EEC, Council Regulation (EC) No 1493/1999, Directive 2000/13/EC, Council Directive 2001/112/EC and Regulation (EC) No 258/97 (OJ L354, 31.12.2008, page 7) [Made some minor amendments relating to the labelling requirements for enzymes used in foods]
- Commission Regulation (EU) No 1266/2010 of 22 December 2010 amending Directive 2007/68/EC as regards labelling requirements for wines (OJ L347, 31.12.2010, page 27) [Provides an extension to the allergen labelling exemption for wine pending further scientific evaluations]
With the following related legislation:
- Commission Directive 2002/67/EC of 18 July 2002 on the labelling of foodstuffs containing quinine, and of foodstuffs containing caffeine [Provides a derogation from Article 6(6) of 2003/13]
- Commission Regulation (EC) No 608/2004 of 31 March 2004 concerning the labelling of foods and food ingredients with added phytosterols, phytosterol esters, phytostanols and/or phytostanol esters [Provides for additional statements when foods contains added substances]
- Commission Directive 2005/26/EC of 21 March 2005 establishing a list of food ingredients or substances provisionally excluded from Annex IIIa of Directive 2000/13/EC of the European Parliament and of the Council (OJ L75, 22.3.2005, page 33) [Providing a listing of ingredients exempted from the allergen labelling rules] [NOTE: This Directive was repealed on 26 November 2007 by Directive 2007/68 (see above) but transtional arrangements allowed products to be labelled according to its provisions until 31 May 2009]
- But see also: Commission Directive 2005/63/EC of 3 October 2005 correcting Directive 2005/26/EC concerning the list of food ingredients or substances provisionally excluded from Annex IIIa of Directive 2000/13/EC of the European Parliament and of the Council [Addition to text of table]
- Commission Directive 1999/10/EC of 8 March 1999 providing for derogations from the provisions of Article 7 of Council Directive 79/112/EEC as regards the labelling of foodstuffs
- Council Directive 90/496/EEC of 24 September 1990 on nutrition labelling rules of foodstuffs. As amended by:
- Commission Directive 2003/120/EC of 5 December 2003 amending Directive 90/496/EEC on nutrition labelling for foodstuffs [Providing a revised energy calculation for a novel food ingredient (salatrims)]
- Commission Directive 2008/100/EC of 28 October 2008 amending Council Directive 90/496/EEC on nutrition labelling for foodstuffs as regards recommended daily allowances, energy conversion factors and definitions [Providing revised/new values for RDAs, providing energy conversion factors for fibre and erythritol and providing a definition of fibre]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods (OJ L404, 30.12.2006, page 9)
- but see also: Corrigendum to Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods (OJ L 404, 30.12.2006) (OJ L12, 18.1.2007, page 3) [Complete replacement of the text - incorrect version published initially]
- Regulation (EC) No 107/2008 of the European Parliament and of the Council of 15 January 2008 amending Regulation (EC) No 1924/2006 on nutrition and health claims made on foods as regards the implementing powers conferred on the Commission (OJ L39, 13.2.2008, page 8) [Amending the procedure for the adoption of specific implementing regulations]
- Regulation (EC) No 109/2008 of the European Parliament and of the Council of 15 January 2008 amending Regulation (EC) No 1924/2006 on nutrition and health claims made on foods (OJ L39, 13.2.2008, page 14) [Inclusion of some missing transitional provisions]
- Commission Directive 2008/5/EC of 30 January 2008 concerning the compulsory indication on the labelling of certain foodstuffs of particulars other than those provided for in Directive 2000/13/EC of the European Parliament and of the Council (Codified version) [Requirements relating to packaging gas, sweeteners and liquorice]
Note that this is a codified version and replaced, with no alterations, the following Directives:
- Commission Directive 94/54/EC concerning the compulsory indication on the labelling of certain foodstuffs of particulars other than those provided for in Directive 79/112 [Requirements relating to packaging gas] (Note that link is to incomplete version of the text) As amended by:
- Council Directive 96/21/EC of 29 March 1996 amending Commission Directive 94/54/EC concerning the compulsory indication on the labelling of certain foodstuffs of particulars other than those provided for in Directive 79/112/EEC [Requirement relating to [Requirements relating to sweeteners] (Note that link is to incomplete version of the text)
- Commission Directive 2004/77/EC of 29 April 2004 amending Directive 94/54/EC as regards the labelling of certain foods containing glycyrrhizinic acid and its ammonium salt [Requirements relating to liquorice]
- Council Directive 89/396/EEC of 14 June 1989 on indications or marks identifying the lot to which a foodstuff belongs. As amended by:
- Council Directive 91/238/EEC of 22 April 1991 amending Directive 89/396/EEC on indications or marks identifying the lot to which a foodstuff belongs [Exemption relating to individual portions of ice cream]
- Council Directive 92/11/EEC of 3 March 1992 amending Directive 89/396/EEC on indications or marks identifying the lot to which a foodstuff belongs [Extension of implementation until July 1992]
Consolidated Texts: As the legislation is amended, the Commission sometimes publishes consolidated versions of the original texts. The following links provide consolidated versions of the above texts:
- Directive 2003/13 - Genreal Requirements [7 August 2009]
- Directive 90/496 - Nutrition Labelling [11 December 2008]
- Directive 89/396 - Lot marking [11 March 1992]
Guidelines: Directive 2000/13 incorporates the requirement (originaly introduced by Directive 97/4/EC) to declare the percentage of certain key ingredients (known as “Quantitative Ingredient Declaration” or “QUID”). To assist in the interpretation of this directive, the Commission published guidelines. These can be found at:
Genetically Modified Foods: Labelling controls on GM foods have been progressively introduced. For current controls, see:
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed [For labelling, see Articles 12 - 14]
See also:- Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC 24
- Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC 24
Background
In January 2008, the Commission published proposals for updating the labelling legislation:
- COM (2008) 0040 (pdf file) - Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on the provision of food information to consumers
The proposed Regulation would replace and repeal the following: Directives 2000/13/EC, 87/250/EC, 94/54/EC, 1999/10/EC, 2002/67/EC, 2004/77/EC and Regulation (EC) No 608/2004.
This figure shows the EU procedure for adopting legislation. The coloured items refer to stages which have been reached.
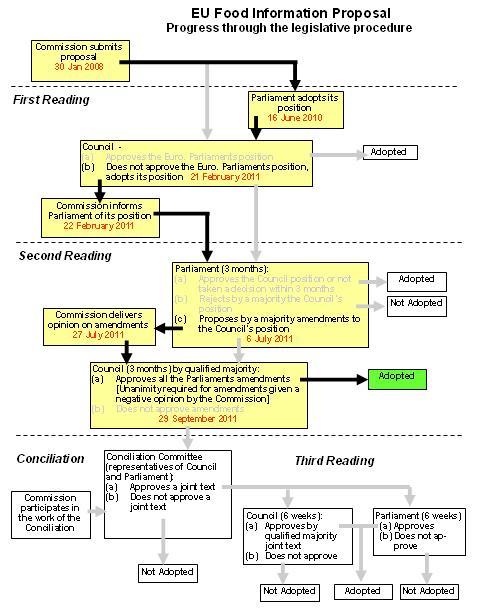
Click here for full size version (pdf file)
Key links to relevant news items will be given here as the legislative process progresses:
- 11 July 2008 European Commission's Proposal for a Food Information Regulation [Council Working Group meeting]
- 14 February 2008 Proposal for a new regulation on the provision of food information to consumers [UK FSA consultation letter]
- 30 January 2008 Commission proposal to overhaul EU food labelling rules [Commission Press Release]
- 30 January 2008 Questions and Answers on Food Labelling [Commission Memo]
- 11 July 2008 European Commission's Proposal for a Food Information Regulation [Council Working Group meeting]
- 15 September 2008 European Commission's Proposal for a Food Information Regulation [Council Working Group meeting]
- 10 October 2008 European Commission's Proposal for a Food Information Regulation - Discussions 30 September & 1 October 2008 [Council Working Group meeting]
- 11 November 2008 European Commission's Proposal for a Food Information Regulation - Discussions 20 October 2008 [Council Working Group meeting]
- 2 December 2008 Presidency's progress report: Document prepared for Council Meeting on 16 December [Council Progress Report]
- 11 January 2010 European Commission's Proposal for a Food Information Regulation - FSA Update on Discussions in Council Working Group meetings and progress report to December Agriculture and Fisheries Council
- 19 March 2009 Council Working Group meetings - 26 January 2009 & 27 February 2009 [Council Working Group meeting]
- 14 May 2009 Council Working Group meetings - 27 March & 27 April 2009 [Council Working Group meeting]
- 23 June 2009 Presidency's progress report: Document prepared for Council Meeting on 22/23 June 2009 [Council Progress Report]
- 30 June 2009 FSA report on Council Working Group meeting (28 May 2009) and Council Meeting (23 June) [Council Working Group meeting]
- 9 December 2009 Presidency Progress Report: Docuent prepared for Council Meeting on 14-16 December 2009 [Council Progress Report]
- 16 March 2010 European Parliament Committee discusses clearer and more informative food labelling rules [European Parliament Committee Report Adopted]
- 7-8 June 2010 Council Discussions: Food information to consumers [Further Council discussions]
- 7 June 2010 European Commission's Proposal for a Food Information Regulation - Update on Discussions in Council Working Group meetings under the Spanish Presidency [FSA letter - update on Council discussions]
- 16 June 2010 MEPs set out clearer and more consistent food labelling rules [European Parliament adopts 1st Reading]
- 22 June 2010 EP Rapporteur Renate Sommer speaks after EP vote
- 7 December 2010 Council agrees on new labelling rules for food [Political agreement on First Reading Position in Council]
- 7 January 2011 Update on Discussions in Council Working Group meetings under the Belgian Presidency [More details on the political agreement]
- 21 February 2011 Council finalizes its first reading on new food labelling rules [Council adopts its “Position”]
- 22 June 2011 Consumers enabled to make healthier dietary choices: Results of discussions between Institutions [Interinstitutional discussions result in compromise text]
- 6 July 2011 Food labels: clearer information for consumers: [Parliament adopts proposal at second reading]
- 6 July 2011 Questions and Answers on the Food Information regulation
- 6 July 2011 Statement by Commissioner John Dalli: The adoption of Food Information regulation is good news for consumers
- 29 September 2011 Council enables consumers to make healthier dietary choices [Council adoption]
The following documents are also available on this site:
- Council Document: Proposal for a Regulation of the European Parliament and of the Council on the provision of food information to consumers – Political agreement [1 December 2010]
- Council Document: Corrigendum to Proposal for a Regulation of the European Parliament and of the Council on the provision of food information to consumers – Political agreement [2 December 2010]
- Council Document: Position of the Council at first reading with a view to the adoption of a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on the provision of food information to consumers. [Adopted by the Council on 21 February 2011; published 28 February 2011]
- Commission Document: COM (2011) 0077 COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT pursuant to Article 294(6) of the Treaty on the Functioning of the European Union concerning the position of the Council at first reading on the adoption of a Regulation of the European Parliament and of the Council on the provision of food information to consumers [22 February 2011]
- Council Document giving some “Questions and Answers” following agreement on a compromise text at the Interinsitutional meeting: Q&A on food labelling and the compromise reached between the Hungarian Presidency, the European Parliament and the Commission [22 June 2011]
- Commission document: COM (2011) 0475 OPINION OF THE COMMISSIONpursuant to Article 294(7)(c) of the Treaty on the Functioning of the European Union,on the European Parliament's amendment[s]to the Council's position regarding the proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILon the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 and repealing Directives 87/250/EEC, 90/496/EEC, 1999/10/EC, 2000/13/EC, 2002/67/EC, 2008/5/EC and Regulation (EC) No 608/2004 [27 July 2011]
- Council Document: LEGISLATIVE ACTS AND OTHER INSTRUMENTS Subject: REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on the provision of food information to consumers [12 September 2011]
- Council Document: ADDENDUM TO “I/A” ITEM NOTE (Brief statements relating to animal welfare information, aspartame, definition of colouring foods and foods pre-packed for direct sale) [16 September 2011]
- Q&A on the new EU food labelling rules [29 September 2011]
UK Law
The Legal Documents
For the United Kingdom (excluding Northern Ireland), the Directive 2000/13 (and the earlier directives) have been implemented into UK legislation by the following Regulations adopted in 1996. [Note that for Scotland and Wales, separate amendments have been made since 2000 but these are not listed here]
- Food Labelling Regulations 1996 (SI 1996 No 1499)
Amendments:- Bread and Flour Regulations 1998 (SI 1998 No 141) [Minor technical amendment only]
- Food Labelling (Amendment) Regulations 1998 (SI 1998 No 1398) [Introduction of QUID and certain other minor changes.]
- Drinking Milk Regulations 1998 (SI 1998 No 2424) [Minor amendments relating to certain definitions involving “milk”]
- Food Labelling (Amendment) Regulations 1999 (SI 1999 No 747) [Introduction of general requirements relating to labelling of GM foods or foods containing GM ingredients.]
- Miscellaneous Food Additive (Amendment) Regulations 1999 (SI 1999 No 1136) [Minor amendment relating to “flour treatment agents”]
- Food Labelling (Amendment) (No. 2) Regulations 1999 (SI 1999 No 1483) [Introduction of certain specific additional requirements relating to QUID.]
- Natural Mineral Water, Spring Water and Bottled Water Regulations 1999 (SI 1999 No 1540) [Minor amendment to defintion of “natural mineral waters”]
- Food Irradiation Provisions (England) Regulations 2000 (SI 2000 No 2254) [Introduction of additional labelling requirements for irradiated foods and ingredients]
- Fish Labelling (England) Regulations 2003 (SI 2003 No 461) [Removal of fish provision from the 1996 Regulations but establishing separate controls]
- Food Labelling (Amendment) (England) Regulations 2003 (SI 2003 No 474) [Introduction of generic name for “meat”]
- Food (Provisions relating to Labelling) (England) Regulations 2003 (SI 2003 No 2647) [Additional requirement for quinine and caffeine]
- Food Labelling (Amendment) (England) Regulations 2004 (SI 2004 No 1512) [Addition of energy factor for salatrims]
- Food Labelling (Amendment) (England) (No. 2) Regulations 2004 (SI 2004 No 2824) [Implementing rules on allergens and deleting the 25% rule]
- The Food with Added Phytosterols or Phytostanols (Labelling) (England) Regulations 2004 (SI 2004 No 3344) [Provides for additional statements when foods contains added substances]
- Food Labelling (Amendment) (England) Regulations 2005 (SI 2005 No 899) [Implementing labelling of certain foods containing glycyrrhizinic acid and its ammonium salt]
- The Food Labelling (Amendment) (England) (No. 2) Regulations 2005 (SI 2005 No 2057) [Providing exemptions to the allergen labelling requirements]
- Food Labelling (Amendment) (England) (No. 2) (Amendment) Regulations 2005 (SI 2005 No 2969) [Correcting the listing of exemptions to the allergen labelling requirements]
- Food Hygiene (England) Regulations 2006 (SI 2006 No 14) [Minor amendment to the definition of meat when used as a generic name]
- Nutrition and Health Claims (England) Regulations 2007 (SI 2007 No 2080) [Minor amendment permitting nutrition and health claims which comply with the EU Regulation on nutrition and health claims]
- Food Labelling (Declaration of Allergens) (England) Regulations 2007 (SI 2007 No 3256) [Adds molluscs and lupins to the list of specified allergens]
- Food Labelling (Declaration of Allergens) (England) Regulations 2008 (SI 2008 No 1188) [Replaces the list of specificed allergens]
- Drinking Milk (England) Regulations 2008 (SI 2008 No 1317) [Updates references to EU legislation in milk definitions]
- Food Labelling (Nutrition Information) (England) Regulations 2009 (SI 2009 No 2538) [Provide a definition of fibre, updates values for vitamin and minerals RDAs and adds some energy conversion factors]
- Food Labelling (Declaration of Allergens) (England) Regulations 2009 (SI 2009 No 2801) [Extends the tranistion period provided in SI 2008 No 1188 for certain allegens using in wine]
- Food Enzymes Regulations 2009 (SI 2009 No 3235) [included a definition of “food enzyme” and made some additional amendments to make it clear that controls for additives also cover enzymes]
- Food for Particular Nutritional Uses (Miscellaneous Amendments) (England) Regulations 2010 (SI 2010 No 295) [Updates the cross-reference to EU legislation to include a new EU Directive]
The labelling of genetically modified foods is controlled by EU Regulation (see above). Enforcement of this is provided by:
- Genetically Modified Organisms (Traceability and Labelling) (England) Regulations 2004 (SI 2004 No 2412)
See also:- Genetically Modified Food (England) Regulations 2004 (SI 2004 No 2335)
Separate, but identical, legislation applies to Northern Ireland:
- Food Labelling Regulations (Northern Ireland) 1996 (SRNI 1996 No 383)
Amendments include:- Food Labelling (Amendment) Regulations (Northern Ireland) 1998 (SRNI 1998 No 253) [Introduction of QUID and certain other minor changes.]
- Food Labelling (Amendment) Regulations (Northern Ireland) 1999 (SRNI 1999 No 143) [Introduction of general requirements relating to labelling of GM foods or foods containing GM ingredients.]
- Food Labelling (Amendment No. 2) Regulations (Northern Ireland) 1999 (SRNI 1999 No 286) [Introduction of certain specific additional requirements relating to QUID.]
- Food Labelling (Amendment) Regulations (Northern Ireland) 2003 (SRNI 2003 No 159) [Introduction of generic name for “meat”]
- Fish Labelling Regulations (Northern Ireland) 2003 (SRNI 2003 No 160) [Revokes Paragraph 1 of Schedule 1]
- Food (Provisions Relating to Labelling) Regulations (Northern Ireland) 2003 (SRNI 2003 No 448) [Additional requirement for quinine and caffeine]
- Food Labelling (Amendment) Regulations (Northern Ireland) 2004 (SRNI 2004 No 266) [Addition of energy factor for salatrims]
- Food Labelling (Amendment No. 2) Regulations (Northern Ireland) 2004 (SRNI 2004 No 469) [Implementing rules on allergens and deleting the 25% rule]
- Food with Added Phytosterols or Phytostanols (Labelling) Regulations (Northern Ireland) 2004 (SRNI 2004 No 515) [Provides for additional statements when foods contains added substances]
- Food Labelling (Amendment) Regulations (Northern Ireland) 2005 (SRNI 2005 No 198) [Implementing labelling of certain foods containing glycyrrhizinic acid and its ammonium salt]
- Food Labelling (Amendment No. 2) Regulations (Northern Ireland) 2005 (SRNI 2005 No 396) [Providing exemptions to the allergen labelling requirements]
- Food Labelling (Amendment No. 2) (Amendment) Regulations (Northern Ireland) 2005 (SRNI 2005 No 475) [Correcting the listing of exemptions to the allergen labelling requirements]
- Food Labelling (Declaration of Allergens) Regulations (Northern Ireland) 2007 (SRNI 2007 No 498) [Adds molluscs and lupins to the list of specified allergens]
- Food Labelling (Declaration of Allergens) Regulations (Northern Ireland) 2008 (SRNI 2008 No 198) [Replaces the list of specificed allergens]
- Drinking Milk Regulations (Northern Ireland) 2008 (SRNI 2008 No 237) [Updates references to EU legislation in milk definitions]
- Food Labelling (Declaration of Allergens) Regulations (Northern Ireland) 2009 (SRNI 2009 No 362) [Extends the tranistion period provided in SRNI 2008 No 198 for certain allegens using in wine]
See also
- Genetically Modified Organisms (Traceability and Labelling) Regulations (Northern Ireland) 2005 (SRNI 2005 No 271)
Guidance Documents
To help compliance with the legal requirements and to provide general advice, the Food Standards Agency (and before it the Ministry of Agriculture, Fisheries and Food (MAFF)) publishes guidance documents. These are available on the FSA web pages (see FSA Labelling Guidance). The following are the main documents:
General Guidance
- Legal requirements: Food Labelling Regulations Guidance Notes (January 1997)
- Best Practice Guide: Clear Food Labelling Guidance Notes (June 2008)
Date Marking
- Use by date Guidance Notes (February 2003)
Marketing Terms
- Criteria for the use of the terms Fresh, Pure, Natural etc in food labelling (July 2008)
- Guidance on the use of the terms “vegetarian” and “vegan” in food labelling (April 2006)
Origin Labelling
- Origin Labelling Guidance Notes (October 2002)
Allergens
- Legal requirements: Guidance notes and best practice on allergen and miscellaneous labelling provisions (July 2008)
- 'May contain' gudiance: Guidance on allergen management and consumer information (July 2006)
Claims (Health/Nutrition)
- Guidance to compliance with European Regulation (EC) No 1924/2006 on nutrition and health claims made on foods (April 2008)
Nutrition Labelling
- Guidance Notes on Nutritional Labelling (December 1999)
Quantitative Ingredient Declarations (QUID)
- The Food Labelling Regulations 1996: Guidance Notes on Quantitive Ingredient Declarations (“QUID”) (March 2003)
Lot Marking
- Lot Marking Regulations Guidance Notes (November 1996)
GM Labelling
- Guidance Notes on Reg (EC) No 1829/2003, GM food and feed, and Reg (EC) No 1830/2003, traceability and labelling of GMOs (August 2007)
This page was first provided on 30 October 2001